Trump announces US withdrawal from World Health Organisation
Trump has long been critical of the WHO, and his administration formally withdrew from the organisation in July 2020 as the Covid-19 pandemic continued to spread.
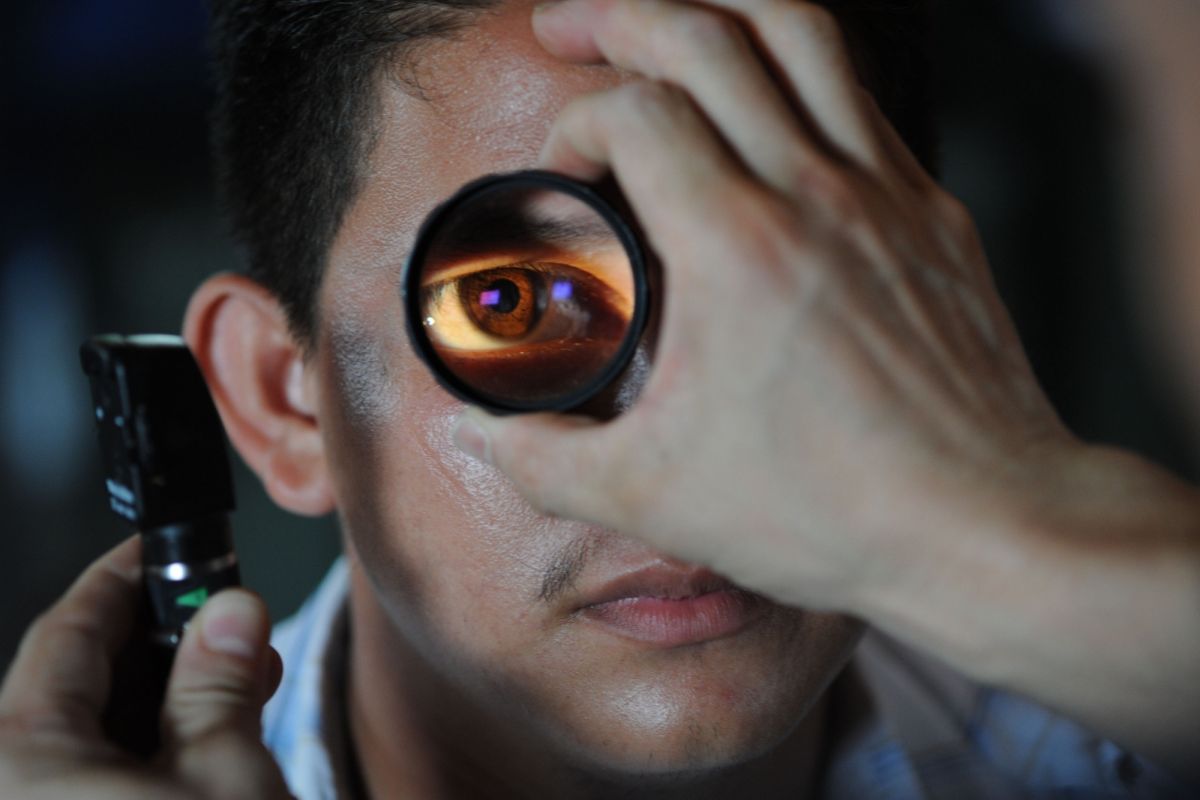
The tear film is a thin fluid layer covering the ocular surface, responsible for the eye’s surface comfort, mechanical, environmental and immune protection, and epithelial health. It also forms a smooth refractive surface for vision, Jerusalem Post reported.
(Photo: IANS)
An Israeli company is conducting a study on a device that measures tear film — a thin fluid layer covering the ocular surface, which may help detect Covid-19 disease through a simple eye test.
The tear film is a thin fluid layer covering the ocular surface, responsible for the eye’s surface comfort, mechanical, environmental and immune protection, and epithelial health. It also forms a smooth refractive surface for vision, Jerusalem Post reported.
Advertisement
The study seeks to determine whether the Tear Film Imager (TFI) device can “effectively diagnose and determine Covid-19 in a fast, affordable, non-invasive assessment of the eye’s tear film”, the company Advanced Optical Methods (AdOM) announced late on Thursday.
Advertisement
“The world urgently needs new diagnostic tools to help assess and diagnose aggressive viruses in a non-invasive manner and with speed and efficiency,” AdOM CTO Raanan Gefen was quoted as saying.
The TFI device is a non-invasive piece of medical equipment designed to simultaneously measure the mucins — proteins that hydrate the tear ducts — and the lipid sub-layers of the eye that prevent the eyes from drying out due to evaporation.
The diagnostic device works at a resolution depth of a few nanometers, and can provide clinicians with a detailed assessment of the health of the tear film sub-layers within just 40 seconds.
The device can also detect when a “virus layer” is present in the eye and can quantify it. As previously observed in a concept study at Wolfson Medical Center, it has been able to correctly identify corona in patients at the same rate as a PCR test, the report said.
“Different SARS variants, as well as aggressive flu variants, are threatening the world population. If proven to have a high correlation to the PCR, this could be a game-changer,” Gefen said.
Gefen noted that the TFI device could then be used as a point of diagnostic care in many venues such as airports, sporting arenas and businesses that want to have a simple, non-invasive test to determine the status of entering crowds.
The new study will expand on the concept study and observe the use of the TFI in comparison to PCR diagnostic testing on a larger scale. The study, expected to take 30 days to complete, will observe 500 patients, the report said.
Advertisement