Sudan clash: Death toll reaches 270, over 2,600 injured
Fighting continued in Sudan as forces loyal to duelling generals fought for strategic positions in the city and accused one another of breaking the ceasefire hours.

Fighting continued in Sudan as forces loyal to duelling generals fought for strategic positions in the city and accused one another of breaking the ceasefire hours.

The states were also asked to focus on timely testing for early identification and ensuring adequate health infrastructure.

Indeed, WHO has upbraided the countries that are asking citizens to take yet another Covid-19 booster shot to mitigate the transmission of the virus.
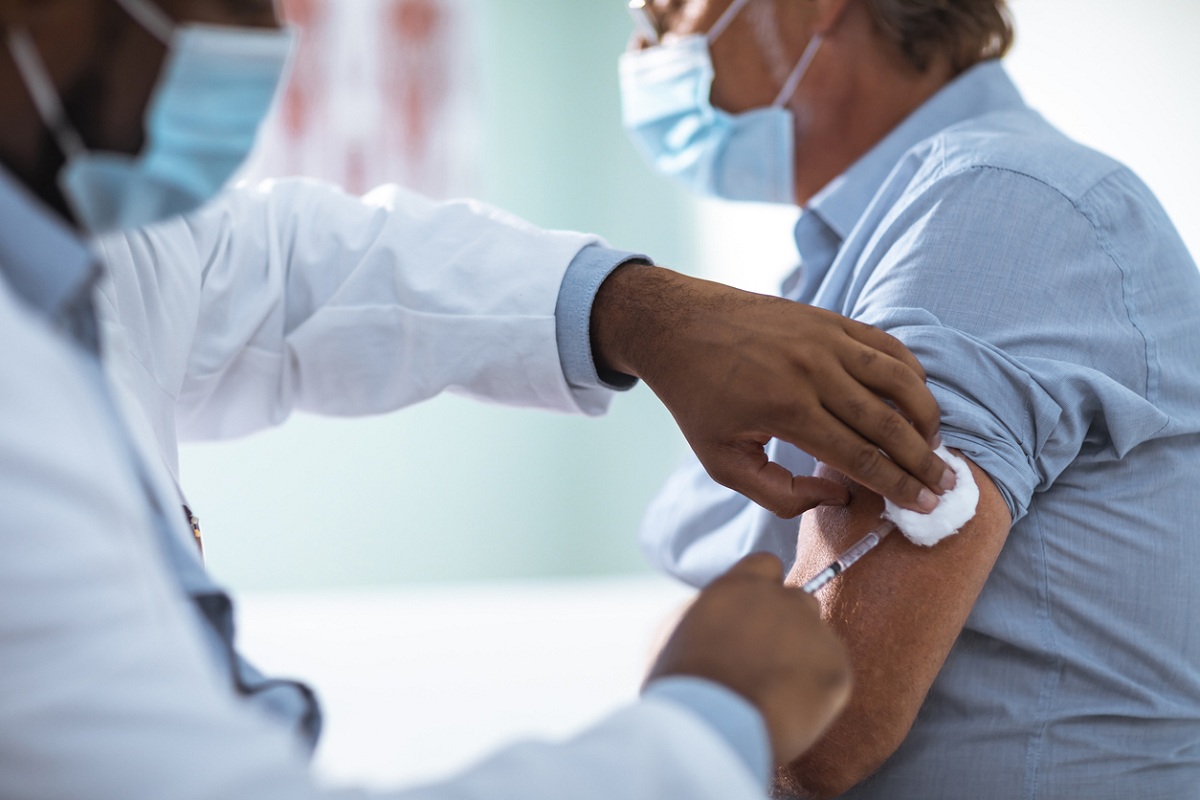
On the International Day of Older Persons, “The older population continues to be highly vulnerable in the ongoing pandemic. Protecting them against the deadly COVID-19 virus should be our priority," said Dr Poonam Khetrapal Singh, Regional Director, WHO South-East Asia Region.

"The Delta variant now accounted for 90 per cent of the sequences submitted to GISAID with a sample collection date (between 15 June-15 September 2021)," the global health agency said in its weekly epidemiological update on Tuesday. GISAID, which stands for Global Initiative on Sharing Avian Influenza Data, is an open-access database.
This piece of news gains significance in light of the Delta variant (B16172) that has now spread to at least 92 countries, hence becoming a dominant variant worldwide.
The members said they strictly follow the Covid protocols by wearing masks, head gears, gloves and using hand sanitisers.
According to experts in the region, the new variant, which is being referred to as the "Vietnamese variant," is far more infectious and can cause more deaths in an unvaccinated population.
While Covishield and Covaxin may not be accepted in many countries, should they not pass muster within India? Yet even fully vaccinated and healthy citizens need a negative RT-PCR report to travel to several states including West Bengal and Punjab.
In December 2020, the US Food and Drug Administration issued an emergency use authorisation for the Moderna vaccine, while the European Medicines Agency granted it a marketing authorisation valid throughout the European Union in January this year.