Rapid antigen tests have reduced sensitivity against Omicron: US FDA
The findings are based on a study conducted by the FDA in collaboration with the US National Institutes of Health (NIH).
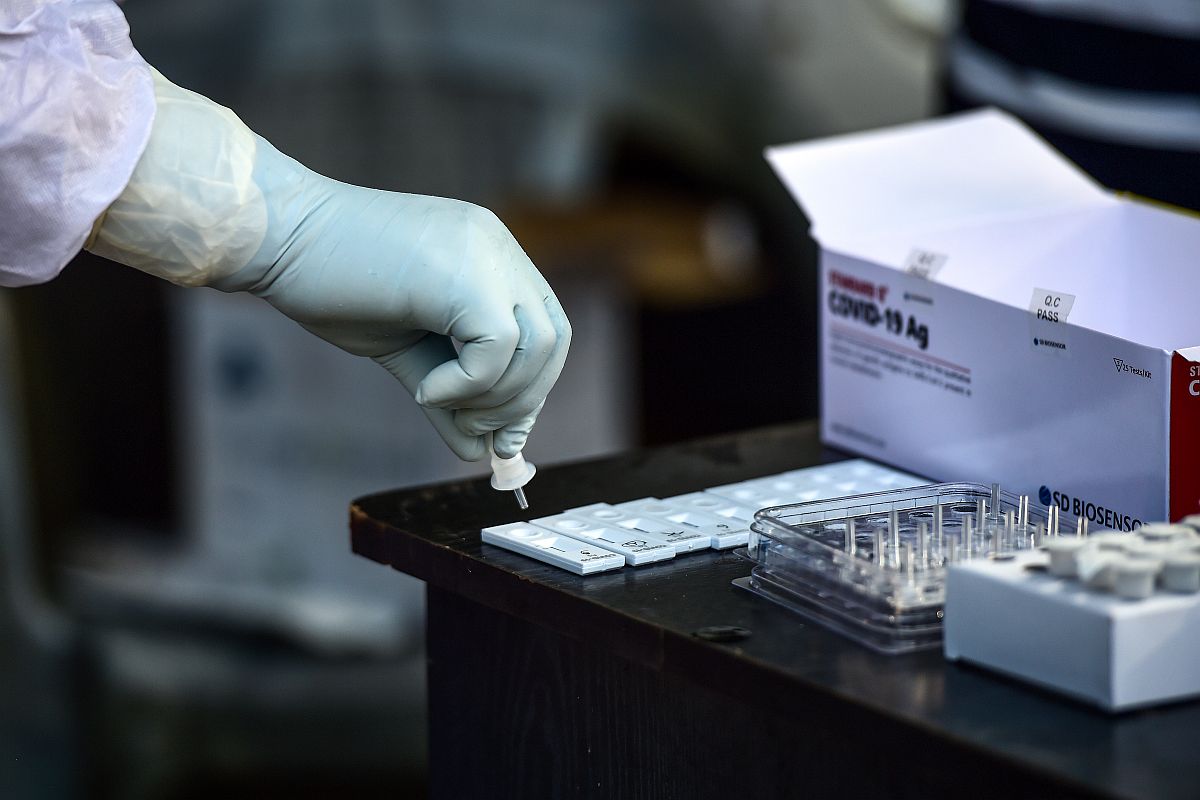
The findings are based on a study conducted by the FDA in collaboration with the US National Institutes of Health (NIH).
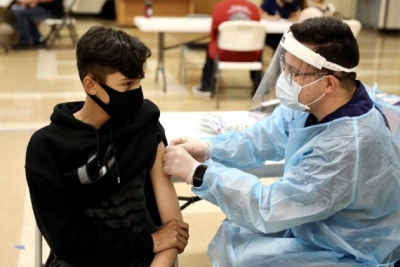
National Institutes of Health Director Francis Collins told Fox News on Sunday that the guidance issued two days ago by the FDA panel is in line with what the US administration planned for a booster rollout.

Members of the committee expressed doubts about the safety of a booster dose in younger adults and teens.

Britain, Canada, Bahrain and Saudi Arabia have already approved the vaccine, the first in the world to complete a large-scale, phase 3 clinical trial.

The green light for drugmaker Regeneron came after REGEN-COV2, a combination of two lab-made antibodies, was shown to reduce Covid-19-related hospitalizations or emergency room visits in patients with underlying conditions.