US FDA delays approval for Moderna vax for teens
Moderna also said it will delay filing a request for emergency use authorisation for a smaller dose of the vaccine for younger kids aged six to 11 while the FDA completes its review.
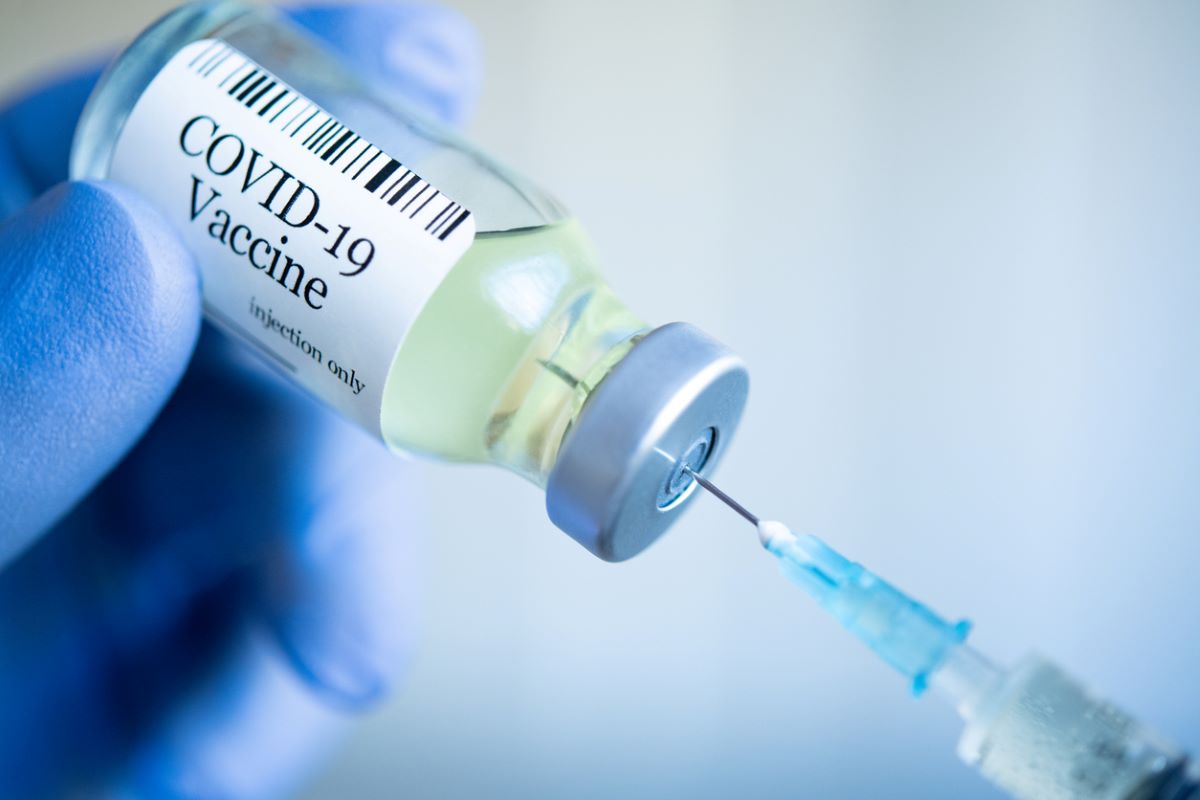
Moderna also said it will delay filing a request for emergency use authorisation for a smaller dose of the vaccine for younger kids aged six to 11 while the FDA completes its review.
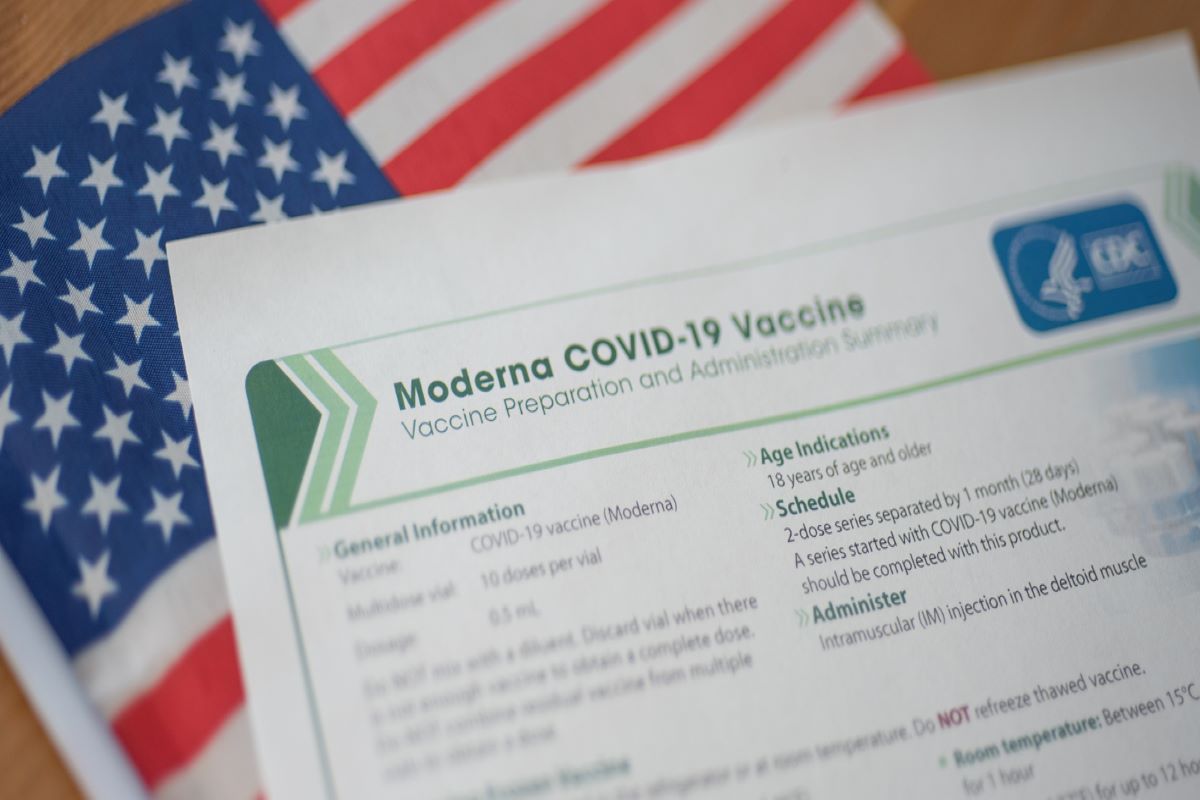
More than 7 million Americans have received a booster dose in the US as of Saturday, according to the latest data available from the CDC.
"We believe our mRNA platform can solve the world's greatest health challenges, from diseases impacting millions, to ultra-rare diseases impacting dozens, to medicines personalised down to the individual level,"

The Minister for Health and Aged Care, said the third Covid-19 vaccine added to Australia's rollout boost the vaccine plan during a tough period.
The findings showed that it induced robust antibody responses against the wildtype D614G Covid-19 strain and against important variants of concern including Gamma (P1); Beta (B1351); and Delta (B16172), the US pharmaceutical major said in a statement.
It is to be noted that the government has not given indemnity to any vaccine companies from severe side effects as of now.
This means that Pfizer and Moderna vaccines will not require clinical trials in India.
The US President has launched what he calls a “new phase” of his pandemic effort.
The Phase 2 trial showed that antibodies produced by a single 50 mg dose of its booster shot was effective against the original form of the virus, as well as against two variants of concern first identified in South Africa (B1351) and Brazil (P1).
The US is the first nation to authorize the two-dose regimen from Moderna, now the second vaccine to be deployed in a Western country after the first, developed by Pfizer and BioNTech.