Why has AstraZeneca recalled Covid-19 vaccine
While the company made the application to withdraw the vaccine on March 5, it came into effect on Tuesday.
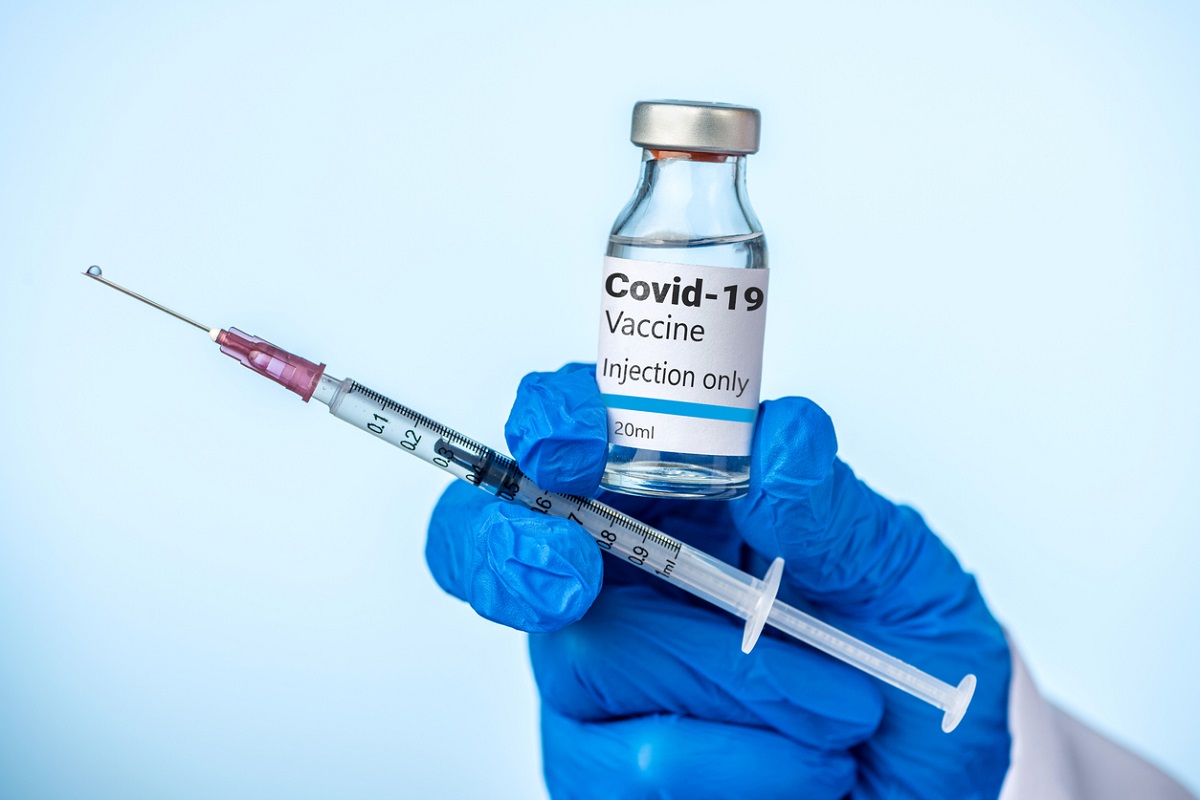
While the company made the application to withdraw the vaccine on March 5, it came into effect on Tuesday.

Addressing a press conference, the Delhi Health Minister raised concerns about the central government's continuing promotion of this vaccine despite such risks, highlighting issues of blood clots and falling platelet counts leading to heart attacks.
Benefits of the Covid-19 vaccine far outweigh the risks of extremely rare potential side effects, pharma giant AstraZeneca said on Tuesday.
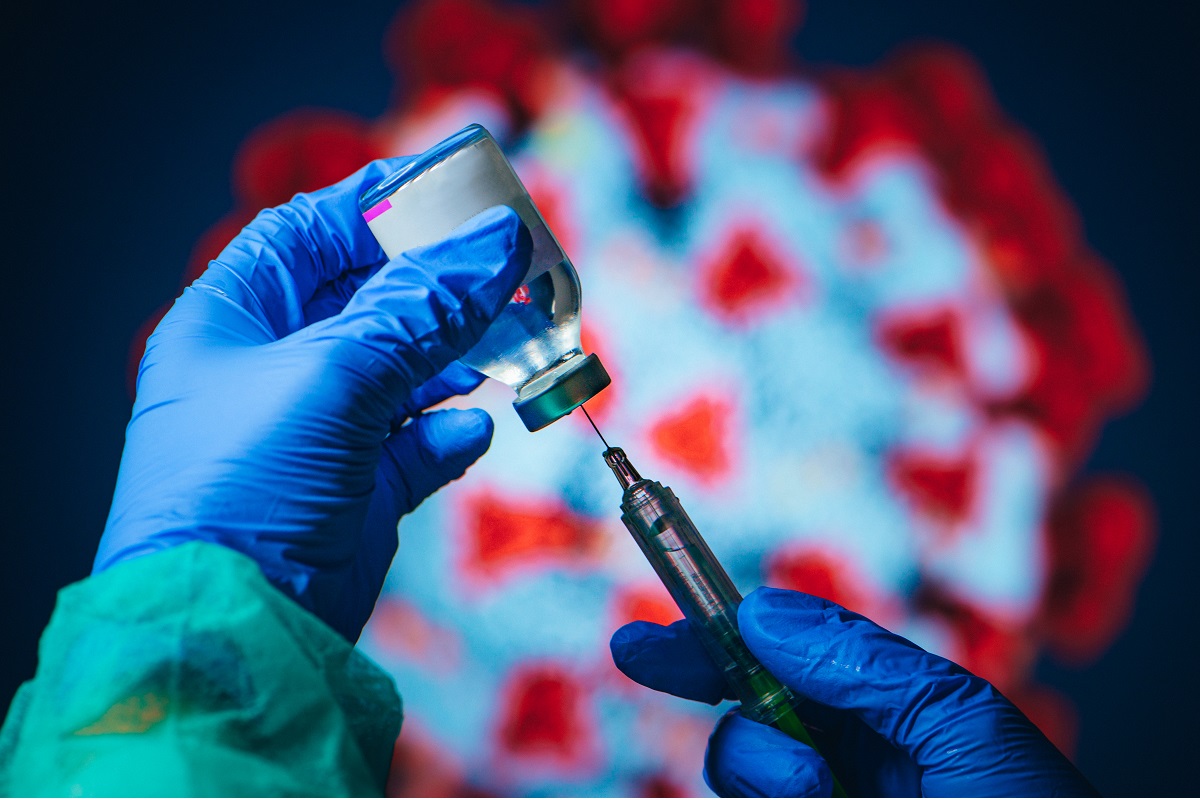
In light of recent concerns surrounding potential rare side effects of the AstraZeneca-Oxford COVID-19 vaccine, the pharmaceutical giant has reiterated its commitment to patient safety while emphasising the vaccine's overall safety profile.
The Covid-19 vaccine misinformation policies of Facebook, the world’s largest social media platform, were not effective in combating misinformation and…
The webinar aimed to make influencers act as catalysts, and to educate and share reliable information with their community of followers. It was held on the occasion of World Social Media Day, celebrated on June 30 each year.
The Pfizer-BioNTech shot has been approved by the US Food and Drug Administration (US FDA) and other western regulators for emergency use. The vaccine is also authorised for use in teens in the US.
An Indraprastha Apollo hospital official told IANS on Sunday that Sputnik V was yet to be rolled out, but "the management is working on it".
This piece of news gains significance in light of the Delta variant (B16172) that has now spread to at least 92 countries, hence becoming a dominant variant worldwide.
The Prime Minister warned, "avoiding vaccine jabs may be very dangerous and such actions will not only push every individual's life in danger but that of the family members as well".