Why has AstraZeneca recalled Covid-19 vaccine
While the company made the application to withdraw the vaccine on March 5, it came into effect on Tuesday.
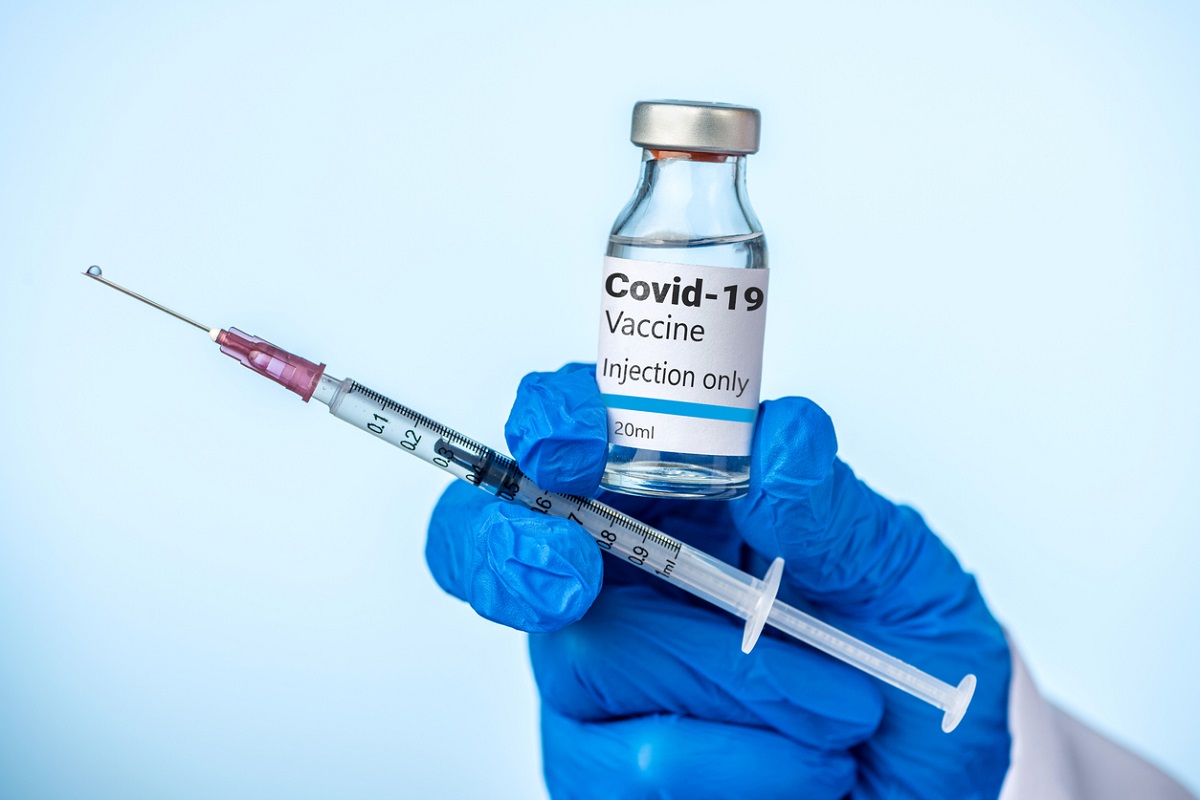
While the company made the application to withdraw the vaccine on March 5, it came into effect on Tuesday.

Addressing a press conference, the Delhi Health Minister raised concerns about the central government's continuing promotion of this vaccine despite such risks, highlighting issues of blood clots and falling platelet counts leading to heart attacks.
Benefits of the Covid-19 vaccine far outweigh the risks of extremely rare potential side effects, pharma giant AstraZeneca said on Tuesday.
In light of recent concerns surrounding potential rare side effects of the AstraZeneca-Oxford COVID-19 vaccine, the pharmaceutical giant has reiterated its commitment to patient safety while emphasising the vaccine's overall safety profile.
The Covid-19 vaccine misinformation policies of Facebook, the world’s largest social media platform, were not effective in combating misinformation and…
The Centre filed this affidavit in response to the plea, filed by Tia Gupta through advocate Bihu Sharma demanding for immediate vaccination of children between the ages of 12-17 in the city, and also prioritizing of vaccination for parents having children up to 17 years of age.
In a tweet earlier in the day, the chairman said that a rapid inflow of infected patients in hospitals as well as in critical care centres were being reported across the country, reports Xinhua news agency.
The move came amid Pfizer's request to the regulators of the US and Europe for approving a booster dose of its vaccine.
According to the state health bulletin, 2,52,07,840 people, including senior citizens above 45 years, healthcare workers, frontline warriors and those in the 18-44 years age group received the jab over the last 6 months.
With this initiative, the Gurugram district will be among the first ones across Haryana, where the Russian-made vaccine will be administered at government facilities.