5 Reasons why you should incorporate peptides in your skincare
Over the past few years, skincare and the patterns related to it have changed significantly.
Ribosomes play a central role in protein synthesis and catalyses formation of the peptide bonds that link amino acids into a polypeptide.
For genes encoding ribosomal ribonucleic acid and transfer RNA (and certain other small RNA molecules), RNA is the ultimate expression of a gene. But for the thousands of other genes in an organism’s genome, the ultimate gene product is protein.
Translation, the first and most important phase of protein synthesis, involves a change in language from the nucleotide sequence of an mRNA molecule to the amino acid sequence of a polypeptide chain. In essence, the sequential order of mRNA nucleotides, read as triplet codons, specifies the order in which incoming amino acids are added to a growing polypeptide chain.
Advertisement
Ribosomes serve as the intracellular sites of the translation process, while RNA molecules are the agents that ensure insertion of the correct amino acids at each position in the polypeptide. We will start by surveying the cell’s cast of characters for performing translation, before examining in detail the steps of the process.
Advertisement
The cellular machinery for translating mRNAs into polypeptides involves five major components — ribosomes that carry out the process of polypeptide synthesis, tRNA molecules that align amino acids in the correct order along the mRNA template, aminoacyl-tRNA synthetases that attach amino acids to their appropriate tRNA molecules, mRNA molecules that encode the amino acid sequence information for the polypeptides being synthesised, and protein factors that facilitate several steps in the translation process.
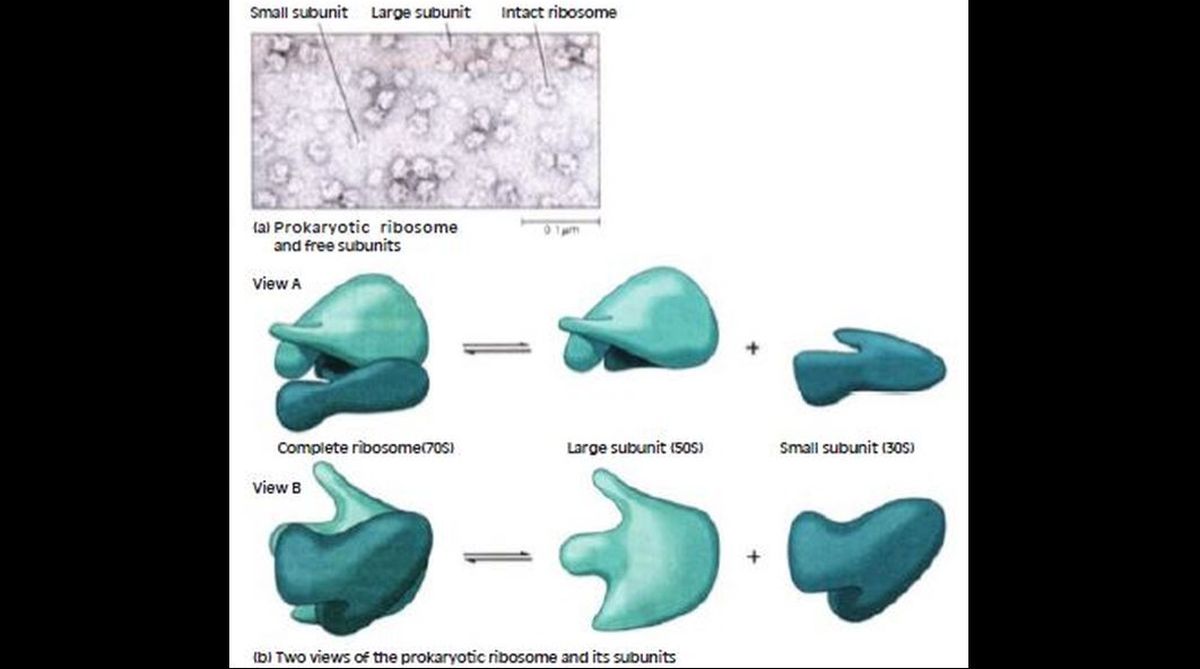
Ribosomes play a central role in protein synthesis, orienting the mRNA and amino acid-carrying tRNAs in the proper relation to each other so the genetic code is read accurately, and catalysing formation of the peptide bonds that link the amino acids into a polypeptide. Ribosomes are particles made of RNA and protein that reside in the cytoplasm of both prokaryotic and eukaryotic cells, as well as in the matrix of mitochondria and the stroma of chloroplasts.
In the eukaryotic cytoplasm, they exist both free in the cytosol and bound to membranes of the endoplasmic reticulum and the outer membrane of the nuclear envelope. Prokaryotic and eukaryotic ribosomes resemble each other structurally, but they are not identical. Prokaryotic ribosomes are smaller in size, contain fewer proteins, have smaller RNA molecules (and one fewer RNA), and are sensitive to different inhibitors of protein synthesis.
Like all ribosomes, prokaryotic ribosome consists of two dissociable subunits called the large and small subunits. A complete prokaryotic ribosome, with a sedimentation coefficient of about 70S, consists of a 30S small subunit and a 50S large subunit; its eukaryotic equivalent is an 80S ribosome consisting of a 40S subunit and a 60S subunit.
Ribosomal RNAs and protein molecules self-assemble into small and large subunits but the two types of subunits come together only when bound to mRNA. X-ray crystallography has recently allowed for the arrangement of all the individual proteins and RNA molecules of small and large subunits of bacterial ribosomes to be pinpointed down to the atomic level.
Functionally, ribosomes have sometimes been called the “workbenches” of protein synthesis, but their active role in polypeptide synthesis makes “machine” a more apt label. In essence, the role of the ribosome in polypeptide synthesis resembles that of a large, complicated enzyme constructed from more than 50 different proteins and several kinds of rRNA.
For many years, it was thought that the rRNA simply provided a structural scaffold for the ribosomal proteins, with the latter actually carrying out the steps in polypeptide synthesis. But today we know that the reverse is closer to the truth — that rRNA performs many of the ribosome’s key functions.
Four sites on the ribosome are particularly important for protein synthesis. These are an mRNA-binding site and three sites where tRNA can bind — an A (aminoacyi) site that binds each newly arriving tRNA with its attached amino acid, a P (peptidyl) site where the tRNA carrying the growing polypeptide chain resides, and an E (exit) site, from which tRNAs leave the ribosome after they have discharged their amino acids.
The writer is associate professor, head, Department of Botany, Ananda Mohan College, Kolkata, and also fellow, Botanical Society of Bengal, and can be contacted at tapanmaitra59@yahoo.co.in
Advertisement