US scientists decode 125-year-old element to boost cancer treatments
US researchers have decoded an element that could destroy cancerous cells, and advance treatment options for the deadly disease that claims millions of lives globally.
Russian chemist Dmitri Mendeleev published what is regarded as the first clear classification of the elements based on a measure of the mass of the atoms, in 1869
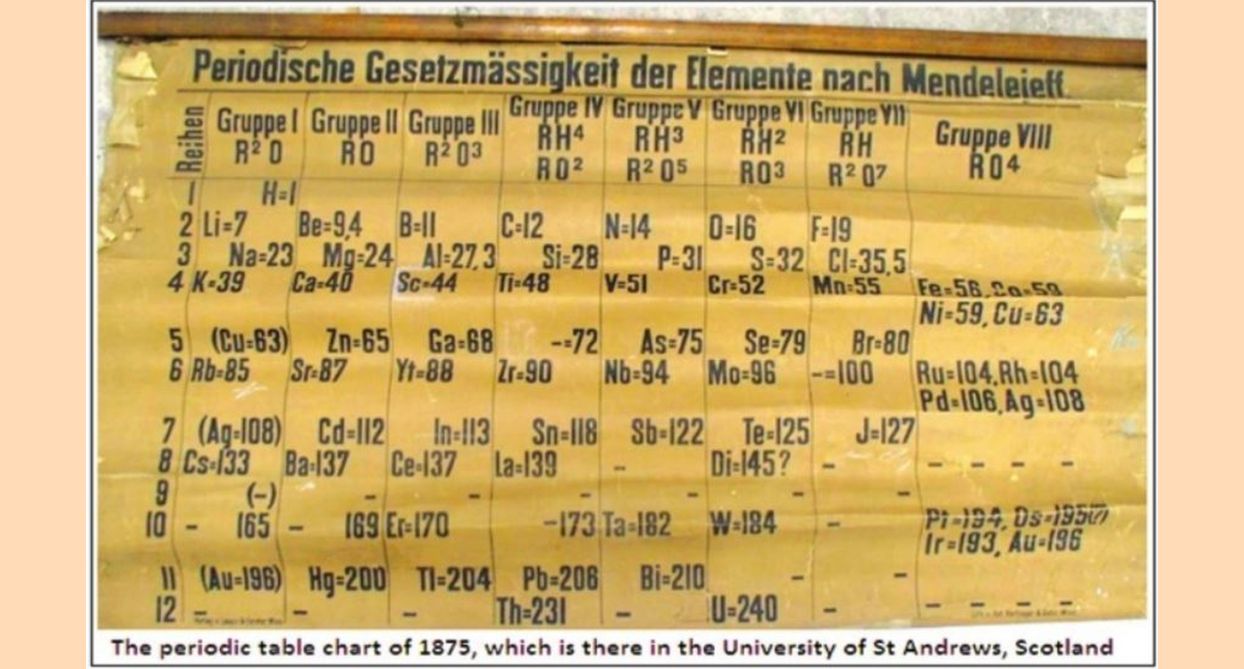
The periodic table of the elements is a piece of insight that laid the foundation for what was discovered about atoms and the nature of matter in the years that followed. It is a sign of the creative wave that swept through the 19th century, that while Dalton had proposed a barebones atomic theory at the start of the 1800s, Dmitri Mendeleev published what is regarded as the first clear classification of the elements based on a measure of the mass of the atoms, in 1869.
Physics World, the magazine published by the Institute of Physics, reports that the University of St Andrews, in Scotland, which houses the 1875 display, the oldest known, of the periodic table of the elements, is conducting special events to commemorate this 150th year. The United Nations has also declared 2019 to be the International Year of the Periodic Table.
Advertisement
The atomic theory of Dalton was a far cry from the understanding of the atom that we have today. Dalton built on the observations that gases, when they combined, seemed to do so in simple ratios of volumes or masses. His own studies had shown that if given quantities of gases, in a container, exerted certain pressures by themselves, then the gases , when placed together in the same container, exerted the sum of the individual pressures.
Advertisement
This suggested that the gases were “porous” or allowed other gases to “pass through”, as if they were crowds of people merging together, and hence that the gases consisted of particles that were very small, compared to the distances that separated them. These particles were considered the smallest division of an element which had the properties of the element. Dalton chose the word, “atom”, to describe these particles, from “atomos”, the Greek word for “uncut”.
The available data on the weights of different elements that went into forming compounds now helped Dalton estimate the ratios of the weights of atoms. He set the weight of the hydrogen atom as “1”. The “atomic weights”, of the elements would then be the number of times the atoms were heavier than the hydrogen atom.
Water had been found to be 85 per cent oxygen, by weight, and 15 per cent hydrogen. This put the weight of oxygen about six times that of hydrogen. Dalton took the numbers to be seven and one and placed the atomic weight of oxygen at seven. We know now that an atom of oxygen combines not with one atom of hydrogen but with two. And the ratio is eight, rather than seven, which puts the atomic weight of oxygen at 16. But Dalton had made a start, and like he had done with oxygen, he estimated the atomic weights of many other elements. At the time, the elements had been classified, in terms of their properties, as gases, metals or non-metals.
Dalton’s theory gave new direction to the study of the chemical properties of the elements. It had been seen that the elements did not always combine as one atom of a metal with one other, but some combined with two or more atoms of another element. The atomic weights of the known elements had been determined accurately, as well as the tendency to combine with different numbers of other elements.
Another feature that had been observed was that many elements formed groups of three elements with similar properties. Lithium, sodium, and potassium, for example, were a group of three soft, reactive metals. And then, in these triplets, the atomic weight of the middle member was nearly the average of the atomic weights of the outer two. Soon, such relationships were found in groups of four and five and more relationships were found in different groups of elements.
An extension of this feature was then noticed, that characteristic properties of elements seemed to repeat as one ascended the order of atomic weights. Arrangements to show this periodicity were devised, based both on atomic weight and the tendency to combine with more or different atoms.
But it was Dmitri Mendeleev, a Russian professor of chemistry, who first published a table that displayed the periodic trends of the elements then known. Mendeleev, and the German chemist, Julius Lothar Meyer, independently, but just after Mendeleev, organised the elements, according to atomic weights, which were accurately known by then, in rows or columns, with elements that showed a cyclical return of properties appearing in the same row, or as now the practice, the same column. Mendeleev used the atomic weight not as an exact indicator of the position of an element in the table, but as a guide, along with other factors. The result was a tabulation that fit observation closely and, what is more important, there were gaps in the table, corresponding to elements still not known, and these elements were later discovered!
What is remarkable is that all this classification was done at a time when very little was known about the atom itself. That the atom had component parts, the electrons, was announced only in 1897. And still, nothing was known of the structure of the atom. It was only in 1911 that Lord Rutherford discovered that the mass of the atom was concentrated at the centre. And only later was it understood that the centre was positively charged, with many, much lighter, negatively charged electrons in orbit around the centre.
Our current understanding of the distribution of electrons of the atom and of how atoms combine readily explains why certain atoms are metals, which combine with the other category, the non-metals. We know that the different elements have successively increasing charge in the nucleus, and hence the number of electrons, which form a series of shells around the nucleus. And it is the filling up of shells, as one moves up the order of elements, and the start of new shells, that accounts for the cyclic repetition of characteristic properties of elements. We also know that the mass of the atom is not a result of only the positively charged particles, but there is nearly an equal number of neutral particles.
The chemical properties of elements thus depend not on the atomic mass, or atomic weight, but on the number of charged particles, called the atomic number. It is because some elements have more or less neutral particles that the periodic table does not strictly follow the sequence of atomic weights, a refinement that Mendeleev was able to make long before its reason was known. But the periodic table of the elements documented a relationship among the elements which guided the course of investigation into the nature of matter and led to the integration of different discoveries.
(The writer can be contacted at response@simplescience.in)
Advertisement