Nonsense mutation
Here's the lowdown on a unique process of cellular translation.
A precursor called Procollagen forms many types of tissue-specific collagens. Given the complexity of a collagen fiber, it is important to ask how such an elaborate structure is generated.
Collagens are responsible for the strength of the extracellular matrix. The most abundant component of the ECM (Extracellular Matrix) in animals is a large family of closely related proteins called collagens, which form fibers with high tensile strength and thus account for much of the strength of the ECM. Considered collectively, collagen is the most abundant protein in vertebrates, accounting for as much as 25 to 30 per cent of total body protein. Collagen is secreted by several types of connective tissue cells, including fibroblasts. Without collagen, cells in these and other tissues would not have sufficient adhesive strength to maintain a given form.
Two defining characteristics are shared by all collagens: Their occurrence as a rigid triple helix of three intertwined polypeptide chains and their unusual amino acid composition. Specifically, coiiagens are high in both the common amino acid glycine and the unusual amino acids hydroxylysine and hydroxyproline, which rarely occur in other proteins. The high glycine con-tent makes the triple helix possible because the spacing of the glycine residues places them in the axis of the helix, and glycine is the only amino acid small enough to fit in the interior of a triple helix.
Advertisement
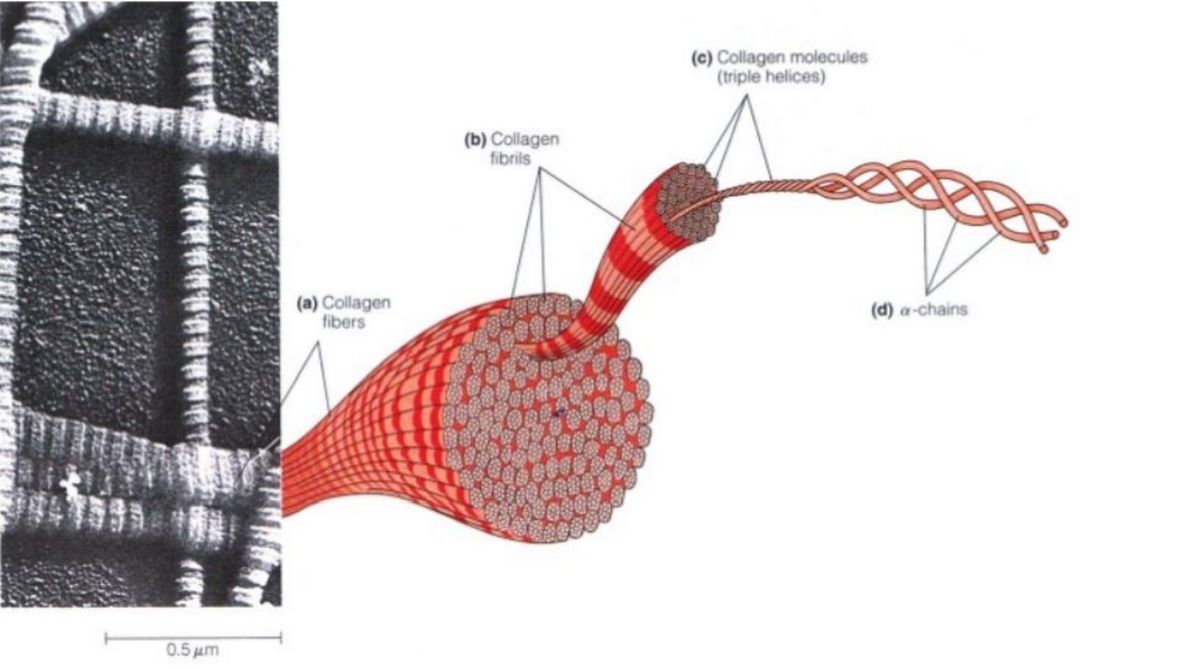
In most animal tissues, collagen fibers can be seen in bundles throughout the extracellular matrix when viewed by scanning electron microscopy. One of the most striking features of collagen fibers is their enormous physical strength. For example, it takes a load of more than 20 pounds to tear a collagen fiber just 1 millimeter in diameter! Not surprisingly, collagen is largely responsible for the mechanical strength of protective and supporting tissues such as skin, bone, tendon, and cartilage. Each collagen fiber is com-posed of numerous fibrils. A fibril, in turn, is made up of many collagen molecules, each of which consists of three polypeptides called chains that are twisted together into a rigid, right-handed triple helix. Collagen molecules are about 270 nm in length and 1.5 nm in diameter and are aligned both laterally and end-to-end within the fibrils. A typical collagen fiber has about 270 collagen molecules in cross section.
Advertisement
A precursor called Procollagen forms many types of tissue-specific collagens. Given the complexity of a collagen fiber, it is important to ask how such an elaborate structure is generated. In the lumen of the endoplasmic reticulum (ER), three a chains assemble to form a triple helix called procollagen. At both ends of the triplehelical structure, short nonhelical sequences of amino acids prevent the formation of collagen fibrils as long as the procollagen remains within the cell. Once procollagen is secreted from the cell into the intercellular space, it is converted to collagen by procollagen peptidase, an enzyme that removes the extra amino acids from both the N- and C-terminal ends of the triple helix. The resulting collagen molecules spontaneously associate to form mature collagen fibrils, which then assemble laterally into fibers.
The stability of the collagen fibril is reinforced by hydrogen bonds that involve the hydroxyl groups of hydroxy-proline and hydroxylysine residues in the chains. These hydrogen bonds form crosslinks both within and between the individual collagen molecules in a fibril. In addition, specialised types of collagen are often present on the surface of collagen fibrils. The triple-helical structure of these specialised collagens is interrupted at intervals, allowing the molecules to bend and hence to serve as flexible bridges between adjacent collagen fibrils or between collagen fibrils and other matrix components.
Vertebrates have about 25 different kinds of a chains, each of which is encoded by its own gene and has its own unique amino acid sequence. These different a chains combine in various ways to form at least 15 different types of collagen molecules, most of which are found in specific tissues.
(Tapan Kumar Maitra is associate professor and head, Department of Botany, Ananda Mohan College)
Advertisement