What are the 8 factors causing you wrinkles?
As we become older, they naturally develop. It is however perfectly normal to have wrinkles on your skin.
A group of scientists writes in the journal, Nature Geoscience, that levels of substances like chloroform are rising anyway, and the improvement in the ozone hole may be slowing down
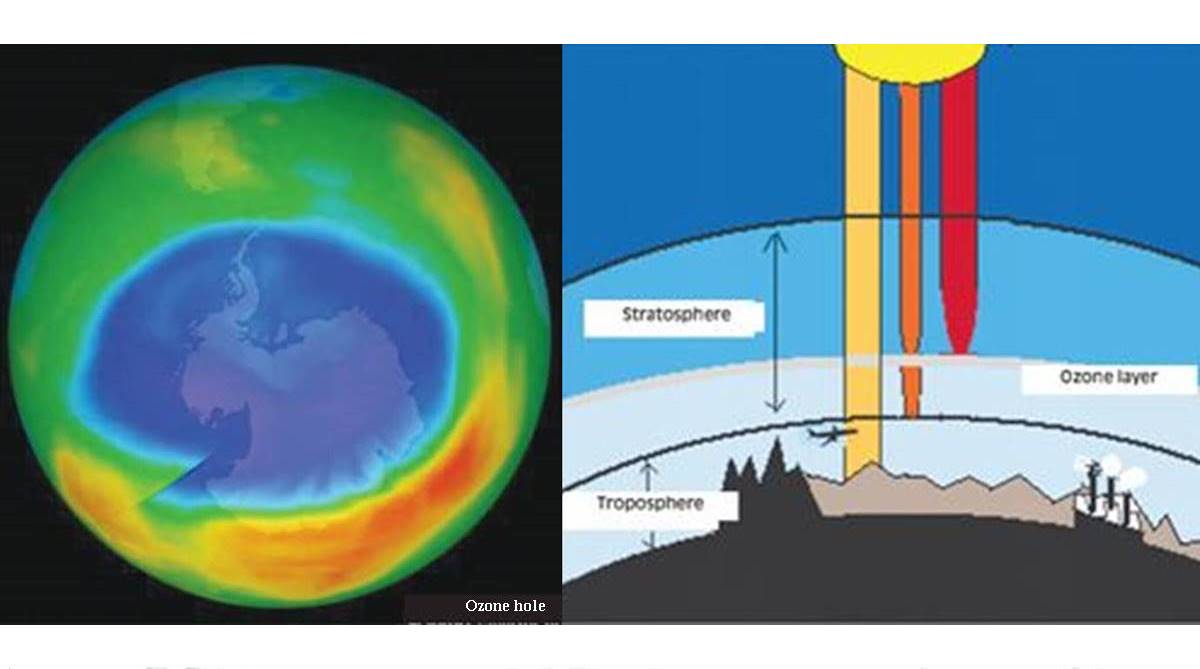
A layer of ozone, high in the atmosphere, protects the earth from ultra violet radiation from the sun. This protective cover had been under attack, but was saved by international action through the Montreal Protocol. The Protocol recognised that manmade chemicals, mainly the chlorofluorocarbons, were depleting the ozone layer, and it set in motion a world-wide drive to cut down the use and release of these substances.
Chlorofluorocarbons, which are contained in refrigerant fluid, in aerosol sprays and industrial solvents, were considered the chief culprit and the focus of campaign was to phase them out. Some other substances, chloroform being one of them, also affect the ozone layer, but as they rapidly decompose, these were not covered under the Protocol. The Protocol has been exceedingly successful and it was expected that the “ozone hole” over the Antarctic would disappear by 2050.
A group of scientists from MIT, the universities of California and Bristol, Kyungpook National University, South Korea, the Climate Science Center at Aspendale, Australia and the Met Office, Exeter, UK, however, writes in the journal, Nature Geoscience, that levels of substances like chloroform are rising anyway, and the improvement in the ozone hole may be slowing down.
Advertisement
Ozone gas is a form of oxygen which builds up at higher altitudes and protects the earth by absorbing much of the ultra violet radiation that comes from the sun.
The oxygen atom has an incomplete outer electron shell and tends to combine with other atoms. Oxygen gas consists of molecules made up of two oxygen atoms that share their outer shell electrons and form a stable unit. At higher altitudes, energetic photons of ultra violet light split oxygen molecules into the component atoms.
Lone atoms represent a higher energy state and they need to form bonds for stability. This, they manage with other oxygen molecules, to form a three-atom molecule of ozone. The ozone molecules again, readily absorb ultra violet light and release ‘lone’ oxygen atoms, which then again combine with oxygen molecules to form ozone, and so on. The cycle keeps going till lone atoms become less frequent, because of lone atoms having come together to form normal oxygen molecules, the state of the lowest energy.
There is thus a process of ozone generation, which starts with ultra violet light splitting oxygen molecules and then reduction of ozone when lone atoms combine, leading to a balance of net ozone content at higher altitudes.
This ozone content keeps up the process of absorbing ultra violet radiation and keeping it away from reaching the surface of the earth. Less ultra violet radiation at the surface is a good thing for humans, and other animals too.
But this comfortable condition of a steady level of ozone is disturbed when substances which speed up the break-down of ozone into oxygen migrate into the upper atmosphere.
The most important of these is the negatively charged, OH part of the water molecule, or the NO part of nitric oxide, or free chlorine or bromine atoms. These substances are able to pull the extra oxygen atoms away from ozone and then release oxygen atoms to form other compounds. These substances then get back to pull oxygen atoms from other ozone molecules – and keep doing this for a long time. Chlorine is the most important of these at high altitudes and a single chlorine atom stays active for as long as two years, reacting with 100,000 ozone molecules before it leaves the cycle.
The natural processes that send ozone depleting substances into the atmosphere are negligible. But still, serious depletion of the high altitude ozone layer has been observed since the 1970s. The cause has then been pinned on the release of chlorofluorocarbons, the CFCs, whose use in industry had begun to rise. These materials, which are volatile, diffuse to the high reaches of the atmosphere and release single atoms of chlorine, which wreck havoc on the ozone layer.
The ozone layer has been there, to contain ultra violet radiation at the surface of the earth, since thousands of years while life forms evolved. Low levels of ultra violet radiation, in fact, may be a precondition for the origin of life. Reduction of ozone and increase in UV radiation hence has serious health implications, one being the increase in incidence of skin cancer. The detection of a region of severe depletion, in the Antarctic, the so called ‘ozone hole’, led to the Montreal Protocol and it has been estimated that checking CFC use would save two million cases of skin cancer by 2030.
Reversal of the trend
As we have said, CFCs were considered the main cause of ozone depletion and the Protocol did not target other causes, like chloroform, both because they are ‘very short-lived substances’ (or VSLS) and because they were considered to arise mainly from natural sources. Nevertheless, the Nature Geoscience paper observes, the level of VSLS in the lower stratosphere has been found to be rising. The level of chloroform the study says, rose from a 3.7 trillionth part in the southern polar atmosphere in 1920 to a 6.5 trillionth part in 1990, and then began to fall. In the northern polar atmosphere, the level rose from a 5.7 trillionth part to a 17 trillionth part over the same period, followed by reduction. The falling trend then continued, at different observation stations, till 2010, but then began to rise. The rise, till the data available for 2015, is mainly in the northern hemisphere. This indicates, the paper says, that the main sources of chloroform entering the atmosphere are in the northern hemisphere.
And further, the magnitude of pollution events, or increases in the chloroform level due to emission from nearby sources, was not significant, during 2007 to 2015, at the observation stations in Australia, the west coast of North America and Europe.
In contrast, there was substantial increase at the stations in Japan and South Korea, during 2010-2015.
To assess why this was happening, the researchers used models to simulate the transport of chloroform from potential sources to the measurement locations. The exercise reveals a rapid increase in emission from eastern China, after 2010, with Japan and South Korea ranking second, and the increase from other East Asian countries being not significant. And the increase in China is in a region that is highly populated and industrialised, consistent with factories that emit chloroform gas. It is clear that a substantial part of chloroform in the air comes from industry and the increase being seen in chloroform levels in man-made, the paper says.
The current level of increase in VSLS, the paper says, could delay the recovery of the ozone hole by several years. “The findings mark an important step towards opening the discussion of regulating anthropogenic VSLS emissions,” says Susann Tegtmeier from the GEOMAR Helmholtz Centre for Ocean Research, Kiel, Germany, in a commentary carried in Nature Geo-science.
(The writer can be contacted at response@simplescience.in)
Advertisement