US engineers develop tiny ID tag that can reveal if an item is real or fake
The earlier tag had a security vulnerability with traditional RFIDs.
Its rays can be harnessed for accelerated evaporation of water and the production of vapour at high temperature.
While it is the sun’s energy that drives all processes on the earth, sunlight itself is usually not a suitable industrial energy source. We need to use “stored sunlight”, as energy found in fossil fuels or indirectly, as the energy in the wind, or in water at higher altitudes, for hydroelectricity. The exception is the case of the solar cell, which uses direct sunlight, but this is a case of considerable complexity.
Thomas A Cooper, Seyed H Zandavi, George W Ni, Yoichiro Tsurimaki, Yi Huang, Svetlana V Boriskina and Gang Chen, from MIT, USA, describe in the journal, Nature Communications, a simple method to harness sunlight to cause accelerated evaporation of water, even production of water vapour at high temperature.
The most visible effect of sunlight is the gentle warmth that causes evaporation. Huge quantities of water from the oceans evaporate and bring about the water cycle, which provides fresh water. Life forms, habitation, even the topography of the earth have evolved because of this effect of sunlight. The freshwater actually available for humans to use, however, is a small fraction, as the bulk is locked as polar ice. With rising demand for water, finding ways to use sunlight for local distillation of seawater or other, impure water has become important.
Advertisement
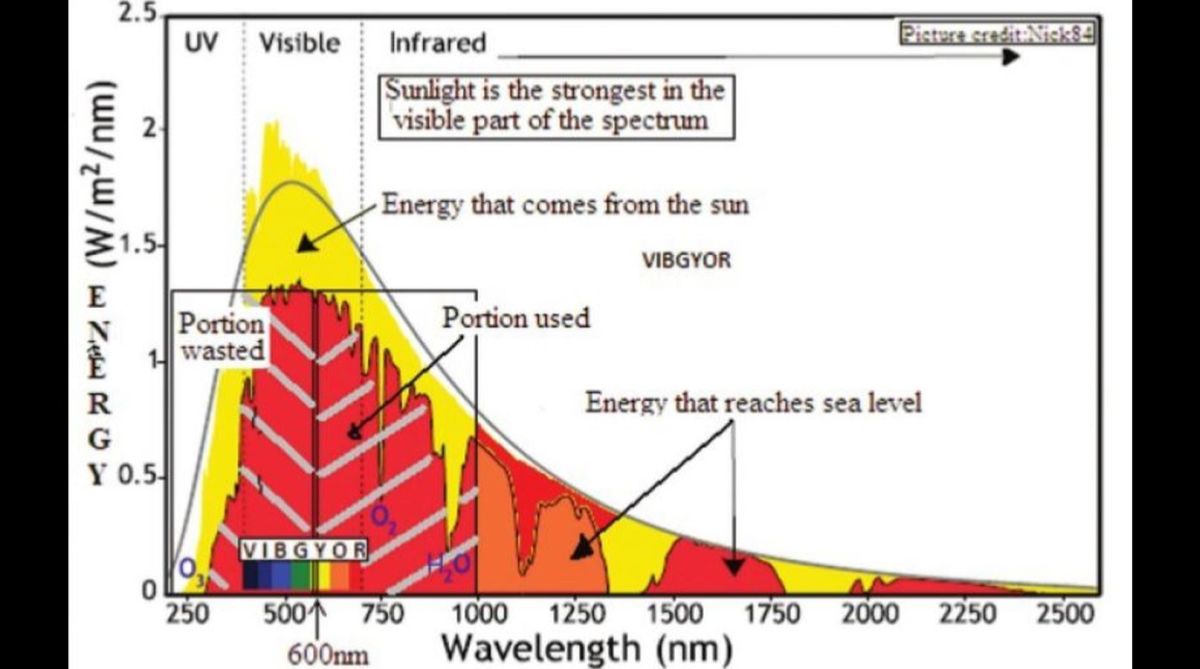
A problem with using sunlight to evaporate water is that water is transparent to the frequencies of light which contain most of the energy in sunlight. Sunlight thus gets absorbed by water only when light penetrates to a depth of 20 to 40 meters, the MIT paper says. There is hence negligible heating of the mass of water and little evaporation. Ways have hence been found to increase the heat absorbed, by introducing particles in the water, or blackening the container.
But with these, the paper says, the water, which is usually brackish water, is in contact with the heat absorbing material, and this leads to deposit of salts or impurities on the material. The material is hence degraded, or corroded, and needs to be cleaned or replaced. There have been developments in materials or structures that can stay clean, but “fouling remains a fundamental challenge inherent to all solar absorbers in direct contact with the water surface”, the paper says.
Another limitation of evaporation using sunlight absorbers that are in contact with water is that the absorber can get only as warm, at best, as the boiling point of water. Sunlight can thus cause evaporation, but it cannot create steam at temperatures above 100°C. While we think of using sunlight for producing potable water in remote places, another need in such places, where there may be no electricity, is a way to sterilize surgical instruments. This application sometimes needs super-heated steam, at 121°C to 135°C which is not possible with solar heaters in contact with water. This has been once done, the paper says, with a composite- material absorber floating on water, but it called for a twenty-fold concentration of sunlight.
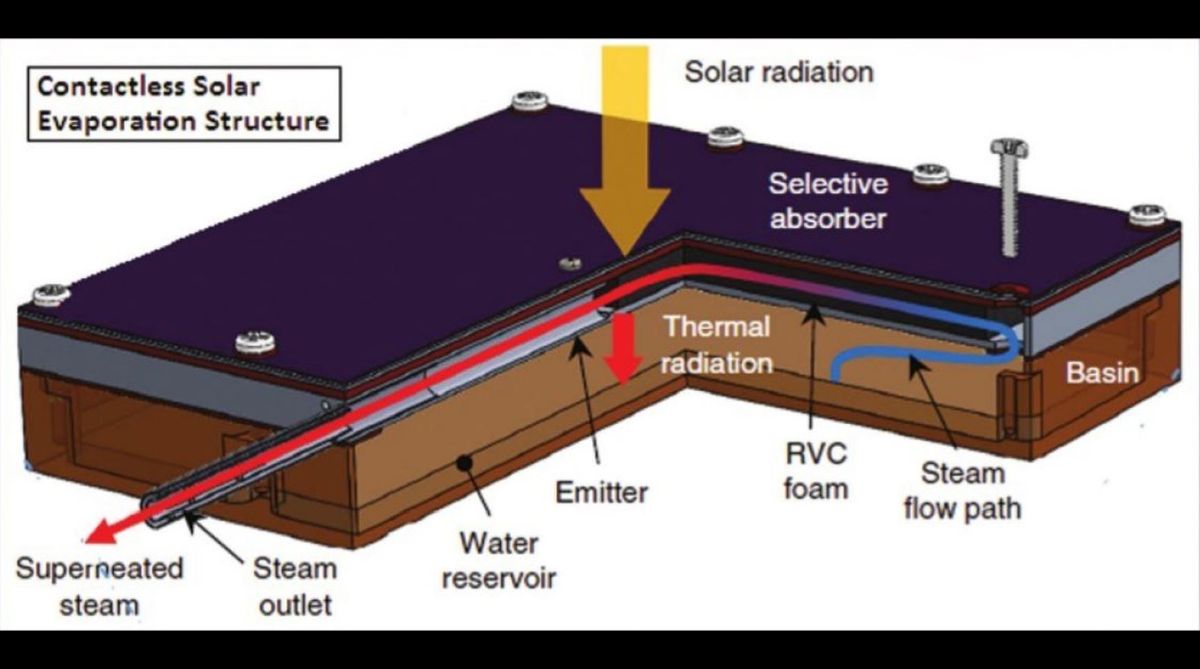
The new approach by the MIT team tackles both the problems with solar evaporation – that water is transparent to sunlight and the need to keep the heating material out of contact with the water. The arrangement is a slab of material that absorbs heat from sunlight, but is not in contact with water, just above the surface of water. While the material warms up in the sun, it radiates heat to the water in the infrared.
The energy coming in from the sun at visible (an ultra violet) frequencies is thus converted to energy in the infrared. Now, unlike in the case of visible light, water is opaque to infrared. 90 per cent of the energy from normal warm objects is absorbed by the top 100 microns (a tenth of a millimeter) of the water, the paper says. Compared to absorption by 20 meters for normal sunlight, this conversion to infrared, which is absorbed at the surface of the water, amounts to a great increase in heating effect.
This naturally increases the rate of evaporation and the efficiency of the arrangement with distillation of bad water, to create exceedingly good quality water, in good quantity, with no power input other than sunlight. But what is more, with the concentrated input of energy into a paper-thin wafer of water, the water need not just warm and vaporise, but can superheat and give off steam at high temperatures. In a demonstration of the device on the rooftop of MIT, water in a reservoir was seen to heat up to its boiling point within an hour and half and superheated steam, at more than 146°C, was generated over three and a half hours, the paper says.
And then, the other feature of the arrangement is that the absorbing radiating material is not in contact with water. There is hence no deposit of salts and impurities, no fouling. To demonstrate the extent of fouling resistance, laboratory experiments were conducted using sea water, which has 3.5 per cent salt content. Even after continuous operation for eight hours, there was no sign of fouling. This is indeed a major advantage, as corrosion and surface degradation are problems that lead to high costs, or water treatment or maintenance, in all technologies where material is in contact with water.
This principle of converting visible sunlight to the more useful, lower frequency, or ‘down-conversion’, has also been applied with ultraviolet light and electric solar cells. While the bulk of the energy in sunlight is in the visible range, there is a considerable part in the higher frequency, and more energetic, ultraviolet. As solar cells are most effective with light at the red end of the spectrum, this higher frequency component of sunlight is lost. What is more, energetic ultraviolet damages and degrades the solar cell material.
In 2015, Jingwen Ding, Jie He and Challa V Kumar, at the University of Connecticut reported a simple material, derived from albumin and coconut fat, as a cover for solar cells, which doubled the output of the solar cells. The principle was a dyestuff that had the property of absorbing energy in the ultraviolet and then emitting the energy at a lower frequency. The result of draping a film of this material on a solar cell was two-fold. First, to convert the energy at high frequencies to a form that the solar cell could use. And second, to use up the high-frequency radiation and protect the solar cell from damage.
The writer can be contacted at response@simplescience.in
Advertisement